Methanol Cracking Process Principle
The mixed superheated steam of methanol and water prepared in a certain proportion passes through the catalyst under certain temperature and pressure conditions, and at the same time, catalytic cracking reaction and carbon monoxide shift reaction occur, and finally a mixture of hydrogen and carbon dioxide is generated.
The methanol water cracking reaction is a multi-component, multi-reaction gas-solid catalytic complex reaction system.
The main reactions are:
CH₃OH <=> CO+2H₂-90.7kJ/mol
CO+H₂O <=> CO₂+H₂+41.2kJ/mol
The overall response is:
CH₃OH+H₂O <=> CO₂+3H₂-49.5kJ/mol
After the reacted mixed gas is heat exchanged, condensed, and separated, a converted gas with a hydrogen content of ~74%, a CO₂ content of ~24.5%, and a CO content of ~0.5% is obtained. The single-pass conversion rate of methanol is more than 95%, and unreacted raw materials (Methanol, desalinated water) return to the raw material system for recycling. The reformed gas is sent to a pressure swing adsorption device for separation and purification to obtain high-purity product hydrogen.
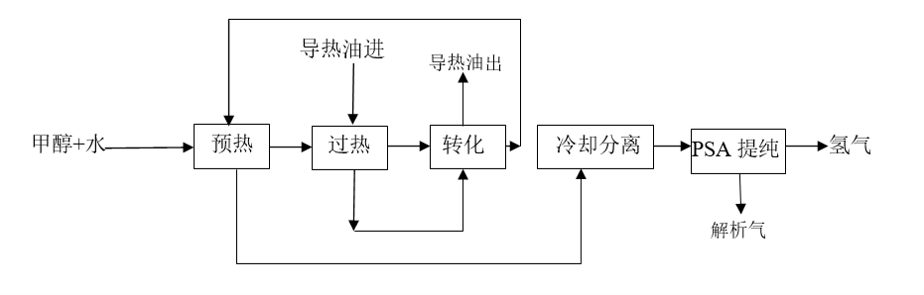
〖Device Characteristics〗
After the methanol and water mixture is preheated, vaporized, and overheated, the catalyst bed reacts to produce hydrogen, carbon monoxide, and carbon dioxide, which are then cooled, separated, and purified by pressure swing adsorption to obtain the product H₂. main feature:
1. The device occupies a small area, is convenient to start and stop, and can produce qualified products in a short time.
2. The use of dual-function catalysts has both conversion and conversion functions.
3. Use a variety of special high-efficiency adsorbents to ensure the quality of hydrogen.
4. The device is equipped with a backup sequence. When the PSA fails, the tower can be cut off to ensure the continuity of production.
〖Performance〗
Yield:50~30000m³/h
Press:0~2.5 MPa
Purity:Up to 99.999%
Unit consumption of methanol:50~60Kg/100Nm³-H₂








